 |
Department of Radiation Oncology |
 |
 |
Department of Radiation Oncology |
 |
| RETURN TO JOHN SOKOL'S HOME PAGE |
INTERSTITIAL RADIOFREQUENCY- INDUCED HYPERTHERMIA
S.D. Prionas, D.S. Kapp, D.R. Goffinet, M.A. Bagshaw,
R. Ben-Yosef,
J.L. Sokol,
and P. Fessenden.
Department of Radiation Oncology, Stanford University
School of Medicine,
Stanford, CA 94305, U.S.A.
ABSTRACT
Continued progress is being made in techniques of heating deep-seated tumors utilizing interstitial radiofrequency RF) currents. while early applications employed rigid, non-insulated metallic trocars, clinical experience highlighted the need for more sophistication allowing for improved control of the local energy deposition. Partially electrically insulated RF electrodes and prototype segmented electrodes are being developed. Preclinical evaluation in static tissue equivalent phantoms illustrates the improved flexibility and control over the three-dimensional (3-D) distribution of energy deposition achievable with these advances. Equipment designed to activate multiple pairs of interstitial RF electrodes is also being developed. Novel hardware features and highly interactive software promise to simplify the control and to improve the system performance. Results of ongoing clinical trials continue to demonstrate the clinical efficacy, safety of application, and utility of this treatment modality. Further development of segmented RF electrodes, hardware and software improvements designed to optimize the delivery and control of RF energy and temperature monitoring, as well as 3-D visualization of the implant promise to significantly improve the ability of this technique to heat increasingly larger implant volumes more uniformly.
Key words: interstitial; hyperthermia; RF; electrodes; segmented;
brachytherapy,
1. INTRODUCTION
The technique of heating deep-seated tumors using interstitial radiofrequency (IRF) currents in conjunction with interstitial brachytherapy (BT) has evolved into a useful clinical tool. The physical basis of this technique is the generation of ionic RF currents flowing between the two electrodes comprising a pair of implantable electrodes, resulting in well localized Joule heating within the implant volume. Since the original introduction of the method by Doss and McCabe (Doss and McCabe, 1976), a number of significant improvements have been made. For example partially electrically insulated stainless-steel trocar electrodes are becoming available. Sophisticated instrumentation to generate, distribute and control the required RF power has been developed. Numerous clinical studies have evaluated the effectiveness in heating tumors located in various anatomical sites, and there is a generalized optimism concerning the clinical utility of this heating approach (Kapp and Prionas, 1992).
The aims of this review are
to discuss some problems related to the physical processes of generation
and control of RF energy deposition, to illustrate the improved capabilities
offered by newly-developed systems, to highlight important contributions
made in the field during the last few years, and to aid in identifying
new directions for future research.
2. INTERSTITIAL RF ELECTRODES
As early as 1976 it was suggested
that RF currents applied between groups of stainless-steel electrodes could
be used to induce elevated temperatures in deep-seated (depth ![]() 3 cm) tumors (Doss and McCabe, 1976). The application of an alternating
voltage of sufficient magnitude across planes comprised of multiple pairs
of such electrodes is capable of generating electrical currents through
the tumor leading to an increase of the tissue temperature. The simplicity
of the basic concept accounts for its increasing acceptance by a number
of hyperthermia research groups, and its application to various anatomical
tumor-bearing sites. In addition, the ability to use the same metallic
trocars for conventional brachytherapy implants has encouraged the clinical
testing of RF interstitial thermobrachytherapy
3 cm) tumors (Doss and McCabe, 1976). The application of an alternating
voltage of sufficient magnitude across planes comprised of multiple pairs
of such electrodes is capable of generating electrical currents through
the tumor leading to an increase of the tissue temperature. The simplicity
of the basic concept accounts for its increasing acceptance by a number
of hyperthermia research groups, and its application to various anatomical
tumor-bearing sites. In addition, the ability to use the same metallic
trocars for conventional brachytherapy implants has encouraged the clinical
testing of RF interstitial thermobrachytherapy
Experience gained from early applications of stainless-steel needle-type electrodes led to the identification of a number of shortcomings and problems. First, potentially cytotoxic elevated temperatures were observed in uninvolved surrounding normal tissues traversed by a portion of these electrodes. This results in pain during treatment and late complications. Second, it soon became apparent that each pair of stainless-steel electrodes implanted in a tumor should be parallel. Converging electrodes can generate temperature hot-spots in the region of closest separation. conversely, cold-spots were observed in the region of greatest separation between a pair of diverging electrodes. Third, the rigidity of the stainless-steel needles occasionally has been implicated as the source of patient pain and discomfort which has become treatment limiting.
In an attempt to resolve some of the intrinsic limitations associated with the use of bare stainless-steel electrodes, we have developed, evaluated and clinically utilized a number of improved IRF electrodes. One such improved electrode consists of a flexible, hollow, partially insulated interstitial catheter, designed to be used as an afterloading catheter for conventional brachytherapy as well as the electrode for inducing local hyperthermia (Kapp, et al., 1988; Goffinet, et al., 1990) More recently, we have been developing implantable multiple-element (segmented) RF electrodes. . These are fabricated through a process of sequential deposition of several coaxial thin films of electrically insulating and electrically conducting materials deposited on the outer surface of a rigid substrate. Preclinical evaluations of these segmented electrodes have demonstrated that they provide flexibility of control over the energy deposition along the longitudinal axis of the electrode. It is anticipated that clinical utilization of these electrodes will result in more uniform temperature distributions in volumetric implants, with improvement shown particularly in the longitudinal direction. In addition, a partially-insulated stainless-steel trocar electrode constructed by selectively coating the outer surface of the metallic electrode with a thin layer of an electrically insulating dielectric material (Polyimide) is also being developed. Our preliminary preclinical and clinical evaluations indicate that this material is effective in preventing current flow through the insulated portion of the electrode and can be used to prevent heating in normal tissue in contact with the insulated portion of the electrode.
One intrinsic limitation
of interstitial heating approaches relates to the inability of the system
to vary the power deposition along the radial direction, i.e. the direction
perpendicular to the electrode length. In general, the power deposition
and the resultant temperatures along the radial direction exhibit a. maximum
located on the surface of the electrode, and usually monotonically decrease
as a function of distance. It is possible, however, to change the radial
temperature distribution by controlling the temperature of the actual RF
electrode. For example it is possible to circulate ternperature-controlled
water through the RF electrode. Then, by adjusting the density of the current
flowing from the RF electrode into the tissue and the temperature of the
water flowing through the electrode, one should be able to reduce the peak
temperature at the electrode surface and improve the uniformity of the
radial temperature profile. A computer simulated study by Prior confirms
this expectation (Prior, 1991).
3. IRF EQUIPMENT
During the last ten years, a variety of hardware configurations have been developed to generate, distribute and control the amount of RF energy needed to raise the temperature of an implanted tumor. The operating frequency has been in the range of 100 kHz to 10 MHz (typically 500 kHz), although operation at even higher frequencies is possible at the expense of increased difficulty in impedance matching, RF interference, departure from mainly I2R Joule heating, etc. In most hardware implementations sonic form of electrode switching is employed in attempts to time-share one, or several, power amplifiers across a large number of RF electrodes. Additionally, some means of adjusting the power level is utilized in order to control and maintain the tumor temperature in the therapeutic range. This control might take the form of amplitude, frequency, or pulse modulation of the carrier signal, and it is exerted either at the stage of preamplification or at the output stage of the power amplifier. More sophisticated hardware implementations configured around the highly versatile architecture of the personal computer (IBM PC/AT) controlled by very elaborate, highly user-friendly control software have recently been developed (Corry, et al. , 1990; Prionas and Kapp, 1992).
At Stanford University, we
have developed and used clinically a 500 kHz heating instrument implementing
electrode multiplexing (Kapp, et al.,. 1988; Goffinet, et al., 1990; Kapp
and Prionas, 1992). An improved second-generation heating device designed
to be used with multiple pairs (up to 32) of partially insulated stainless-steel
trocars or other interstitial RF electrodes has also been developed (Kapp
and Prionas, 1992). A 2x64 computer-controlled matrix is implemented to
provide dynamic electrode multiplexing among 64 single element electrodes,
or alternatively, among 32 dual-element, or 16 quad-element segmented electrodes.
This dynamic computer controlled multiplexing exerts significant control
over the 3-D distribution of energy deposition. Temperature measurements
using copper/constantan thermocouples are taken from temperature sensors
located within each electrode and at present are used to provide the necessary
feedback for automated computer control. Additional thermometry is carried
out using non-perturbing temperature probes mapped within interstitial
catheters placed exclusively for temperature monitoring information. This
is used to document the actual temperature distributions induced in the
target volume. We have also developed interactive software to operate the
heating device, and to acquire, display, and store temperatures, RF power
levels and other treatment parameters useful in later processing and analysis.
Performance evaluation of this heating device in static and pseudo-dynamic
tissue-equivalent phantoms using single and multiple-element electrodes
demonstrated the increased functionality and flexibility in control offered
by electrode multiplexing.
4. RESULTS
4.1 Preclinical evaluation of IRF electrodes
The most fundamental parameter which is used to characterize the operation of a hyperthermia applicator relates to the power deposition, termed specific absorption rate (SAR), generated in the proximity of the applicator (Kapp, et al., 1988). In the case of IRF electrodes, we have used an infrared camera-based technique to measure 2-D distributions of temperature differences induced in a static tissue equivalent two-part split phantom by pairs of parallel IRF electrodes. An example of a 2-D SAR distribution calculated from these data is shown in Figure lA. A pair of partially-insulated IRF electrodes was placed at the surface of the phantom, in contact to a thin sheet of silk screen which defined the measurement plane. The total length of each needle-type electrode was 150 mm. The tips were located at distance X=70 mm (Fig. lA). The first 60 mm from the tip of each electrode (X=10-70 mm; Fig. lA) was the active element (bare stainless-steel), while the remaining length of the trocar was coated with a thin (thickness - 10 urn) film of- Polyimide. A small part of this length can be seen in Figure 1 (L1; X=0-10 mm). RF power was applied (50 Watts) to the pair of electrodes over a period of 10 sec. The infrared image was acquired approximately 2 Sec after power off. Panel A displays the 2-D SAR distribution plotted in the form of iso-SAR contour lines. Notice the absence of power deposition in the electrically insulated area of each electrode. Three additional linear SAR profiles measured along axes A-A', B-B' and C-C' ate shown on panels B, G, and D, respectively. These profiles were normalized to the maximum SAR value observed in the 2-D SAR distribution shown in Fig. lA. Figure lB shows a profile along the direction perpendicular to the two electrodes. Notice the characteristic bimodal distribution. Experimental variability accounts for the asymmetry between the peaks at x=15 mm, and x=25 mm. Finally notice the characteristic form of the longitudinal SAR distribution shown in Fig. 1D. The higher current density at either end of each electrode segment leads to the SAR peaks located at X=10 mm and x=70 mm (Fig. 1D).
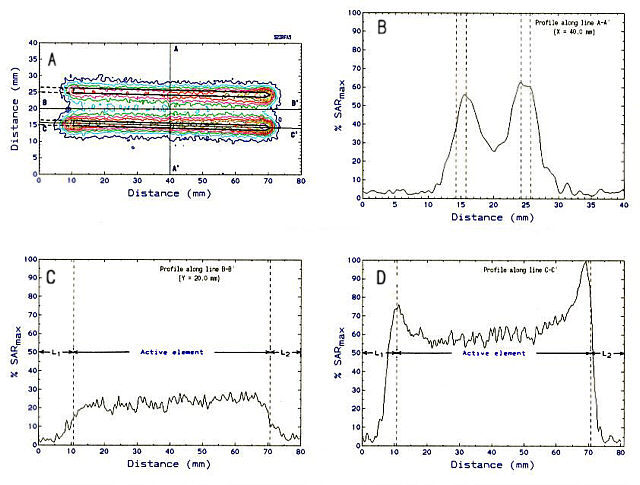
Figure 1. Two-dimensional distribution of relative SAR generated
by a parallel pair of partially-insulated IRF electrodes. Length of active
element = 60 mm; electrode diameter = 1.5 mm; inter-electrode separation
= 10 mm; power = 50 W.
An example of a 2-D SAR distribution generated by a pair of segmented (four elements per electrode) IRF electrodes is shown in Figure 2A. The length of each of the four electrode segments was 14 mm, and the total length of the active zone was 60 mm. Each electrode had a diameter of 2.4 mm, and the inter-electrode distance was 10 mm. RF power levels of 60 Watts were applied across the first and the fourth pairs of electrode segments over a period of 10 Sec. No power was applied across the second or the third pairs of electrode segments. As a result, there is no power deposited along the A-A' axis (Fig. 2B). However, the SAR distributions along the B-B' axis (Fig. 2C) as well as along the C-C' axis (Fig. 2D) demonstrate the ability of. the segmented electrode to modulate the power deposition along the longitudinal axis of the electrode. We obtained very good qualitative agreement between these experimental results and computer simulated 3-D SAR distributions.
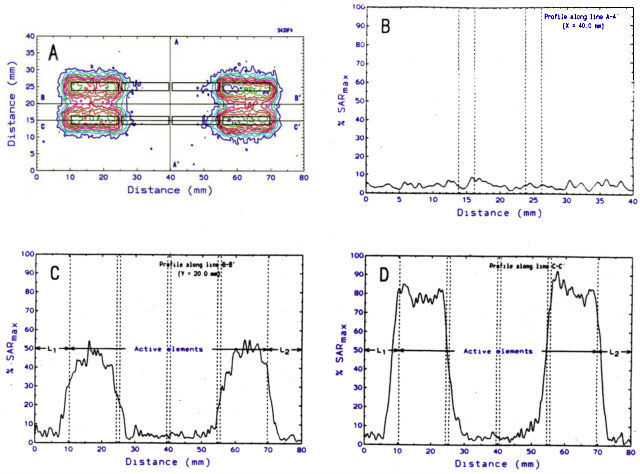
Figure 2. Two-dimensional distribution of relative SAR generated
by a parallel pair of segmented IRF electrodes. Number of segments/electrode
- 4; length of each segment - 14 mm; electrode diameter = 2.4 mm; inter-electrode
separation - 10 mm;inter-segment gap - 1 mm; RF power levels: P1
= P4 = 60 W; P2 = P3 - 0 W.
4.2 Clinical evaluation
We have evaluated the utility
of the IRF hyperthermia system used in conjunction with implantable flexible
afterloading catheters. These catheters were designed so that plastic-encapsulated
ribbons of Ir-192 seeds could be introduced and positioned inside the hollow
. catheter to irradiate the tumor volume. Two HT treatments were given
the first prior to, and the second at the end, of the brachytherapy treatment.
The afterloading catheters were used as RF electrodes for these heating
sessions. In a pilot study of ten patients with carcinomas located in the
head & neck or pelvic regions, we delivered combined interstitial treatments
of brachytherapy and interstitial RF-induced hyperthermia (Goffinet, et
al., 1990). The clinical results have been very encouraging (Kapp and
Prionas, 1992).
Encouraged by the early
success associated with the clinical implementation of the protocol employing
brachytherapy pluS interstitial RF-based hyperthermia, we focused our attention
to other sites. Since 1987 we have evaluated the utility of a similar treatment
protocol applied to patients with locally advanced or recurrent carcinoma
of the prostate (Bagshaw, et al., 1992). The number of partially
insulated stainless-steel RF electrodes used In these implants varied from
7 to 32, depending on the volume of the implant. Extensive thermal dosimetry
employing fiberoptic probes mapped in two-or three thermometry catheters
was carried out. The results of a preliminary analysis of these temperature
maps generated during the treatments of our initial 36 patients (71 treatments)
are summarized in Table I.
Table I: Summary of thermal parameters recorded in 71 IRF treatments of 36 patients with carcinoma of the prostate.
| Thermal Parameter | Value |
| Mean value of minimum mapped intratumoral temperature
Mean value of average mapped intratumoral temperature Mean value of maximum mapped intratumoral temperature Mean value of minimum mapped normal tissue temperature Mean value of average mapped normal tissue temperature Mean value of maximum mapped normal tissue temperature Percentage of mapped intratumoral temperatures Percentage of mapped intratumoral temperatures Percentage of mapped intratumoral temperatures Percentage of mapped normal tissue temperatures Percentage of mapped normal tissue temperatures Percentage of mapped normal tissue temperatures |
38.9 °C
41.9 °C 45.7 °C 37.7 °C 39.8 °C 42.9 °C 68.7 % 46.6 % 27.6 % 21.3 % 9.9 % 3.7 % |
We have also initiated a pilot study to evaluate the use of combined
IRF hyperthermia and interstitial therapy in the treatment of advanced
carcinomas of the vagina or uterine cervix. Ten treatments (five patients)
have been administered to date without significant acute toxicity or complications.
5. DISCUSSION AND CONCLUSIONS
The efficiency of the interstitial RF-based heating technique is a consequence of its simple principle of operation. The choice of a rather low frequency of operation (typically 500 kHz) further facilitates the design of the equipment required for a complete system. The development of partially-insulated RF electrodes is helping significantly to reduce the temperatures in surrounding normal tissues, thus improving the therapeutic gain. Multiple electrode multiplexing also provides added flexibility and the means for dynamic control of power deposition during IRF treatments. The ability of newly-developed control capability allowing modification of the target/goal temperature of each IRF electrode individually can be used to further optimize the delivery of the heat treatment.
Many questions and issues of fundamental importance must be addressed to permit further progress. For example, what is the optimal spacing between the heating electrodes? Is this spacing tissue- or site-dependent? Have we optimized the timing of administration of the brachytherapy and the hyperthermia treatments? Is a single hyperthermia treatment as effective as two IRF treatments? When should the IRF treatment be given in relation to the brachytherapy? Is truly simultaneous heat and ionizing radiation advantageous? etc. These questions and others lead to several areas of research. Perhaps the most important issues at this time from a practical point of view, relate to specific improvements of the instrumentation. It is clear that further development of rigid and flexible segmented RF electrodes is warranted. The potential of warm water flowing through the RF electrode as a means of modifying the radial temperature distributions should be investigated further. Coordinated attempts to improve the data acquisition, display, and 3-D visualization of the enormous amount of information generated during even a single treatment (and needed for making optimal decisions) should be encouraged.
ACKNOWLEDGMENT
This work was supported in part by Contract CM-17480 and Grant CA-44665
awarded from the National Cancer Institute. We acknowledge Richard Cox
and Walter Cox for their help with data analysis.
REFERENCES
Bagshaw, MA., Ben-Yosef, a., Fessenden, P., Goffinet, D.R., Kapp, D.S., Lohrbach, A.W., Mariscal, J.M., Peters-Brown, A.N., Prionas, S.D., Srnitt, MC., and Sokol, J.L. Combined interstitial irradiation and hyperthermia in the treatment of prostatic cancer. Proceedings of the 6th ICHO, Tucson, AZ, 1992.
Corry, PM., Martinez, A.A., Armour, E.P., and Edmundson, G. Simultaneous hyperthermia and brachytherapy with remote afterloading. In: Brachytherapy HDR & LDR, edited by A.A. Martinez, C.T. Orton, and R.F. Mould. Proceedings of meeting entitled "Remote afterloading: state of the art", May 4-6, 1989. Nucletron Corp., publ., Columbia, MD, 1990, pp. 193-204.
Doss, J.D., and McCabe, C.W. A technique for localized heating in tissue: an adjunct to tumor therapy. Ned. Instrum. , 10:16-21, 1976.
Goffinet, DR., Prionas, S.D., Kapp, n.s., Samulski, TV., Fessenden, P., G.M., Lohrbach, A.W., Mariscal, J .M., and Ragshaw, MA. Interstitial 192Ir flexible catheter radiofrequency hyperthermia treatments of head and neck and recurrent pelvic carcinomas.Int. J. Radiat. Oncol. Biol. Phys. ,18(1) :199-210, 1990.
Kapp, D.S. and Prionas S.D. Experience with radiofrequency - local current field interstitial hyperthennia: biological rationale, equipment development, and clinical results. In: Interstitial Hyperthermia, edited by L. Handl-Ze1ler, Springer-Verlag, Vienna, 1992, pp. 95-119.
Kapp, n.s., Pessenden, P., Sarnuiski, TV., Bagshaw, MA., Cox, R.S., Lee, E.R., Lohrbach, AW., Meyer, J.L., and Prionas, S.D. Stanford University Institutional Report: Phase I evaluation of equipment for hyperthermia treatment of cancer. Int. J. Hyperthermia 4(1):75-115, 1988.
Prionas, S.D. and Kapp, D.S. Quality assurance for interstitial radiofrequency-induced hyperthermia. In: Interstitial Hyperthermia, edited by L. Handl-Zeller, Springer-Verlag, Vienna, 1992, pp. 77-94.
Prior, M.V. A comparative study of RF-LCP and hot-source interstitial hyperthermia techniques. Int. J. Hyperthennia, 7(1):131-140, 1991.